Bivalent vaccine
The bivalent vaccines are now being offered at select pharmacies and drug stores but are anticipated to be more widely available soon says CNBC. The bivalent vaccines which we will also refer to as updated boosters contain two messenger RNA mRNA components of SARS-CoV-2 virus one of the original strain of.

Bivalent Covid 19 Vaccine Carecentral Urgent Care Massachusetts
COVID-19 vaccines can help protect against severe illness hospitalization and.
/cloudfront-us-east-1.images.arcpublishing.com/gray/DFXC4VVIYJHHZLYDRKPUJI7CBA.jpg)
. 2 days agoA bivalent vaccine is a Covid-19 vaccine booster that targets both the original strain of Covid-19 and the Omicron variant offering broad protection against Covid-19 and. A bivalent COVID-19 vaccine could also be known as an updated vaccine. Can bivalent vaccines be used as an initial course of vaccination.
This bivalent vaccine is composed of an equivalent mixture of two spike-encoding mRNAs which includes 25 μg targeting the ancestral SARS-CoV-2 strain and 25 μg targeting. Health Canada has approved Pfizers new bivalent COVID-19 vaccine that contains mRNA from both the original SARS-CoV-2 virus and the Omicron BA4 and BA5. For the foreseeable future any adult starting a COVID-19 vaccination.
A bivalent vaccine elicits an immune response against two different antigens. The Moderna bivalent vaccine will be. At this time however the bivalent vaccine is considered only a booster not a means of primary vaccination.
The bivalent vaccines contain two messenger RNA mRNA components instead of one. The new bivalent COVID-19 vaccine includes mRNA from the original strain of SARS-CoV-2 just like the initial vaccine and contains an mRNA component from the. As of Sept.
Adults in the study previously received a Moderna vaccine primary series and one Moderna booster dose. 1 the Centers for Disease Control and Prevention authorized an updated COVID-19 vaccine with the goal of fending off a surge in cases this fall and winter. The bivalent booster combines the original vaccine and a reformulation targeting a mutated spike protein found on the omicron BA4 and BA5 variants so the immune system.
As part of the trial they were given a 50-microgram µg fourth dose of. Adverse reactions following administration of the bivalent vaccine Original and Omicron BA1 as a second booster dose included pain at the injection site 581 fatigue. Who will be eligible.
Theoretically yes but you would need two doses if it is the initial course of vaccination. The bivalent vaccine which Moderna has said it hopes will be authorized for use in the United States this fall is designed to target both the original omicron variant. This can means two different viruses or two variations of one virus.
It is the second. The Pfizer-BioNTech vaccine is authorized for use as a. A bivalent COVID-19 vaccine may also be referred to as updated COVID-19 vaccine booster dose.
More than 200 million Americans are eligible for a bivalent COVID-19 vaccine booster. Vaccination with the bivalent Covid-19 mRNA booster vaccines has began. As with the original.
One of the components is from the original strain of the coronavirus while the other. The latest Omicron COVID-19 vaccine may lead to similar side effects caused by earlier versions which include injection site pain fatigue fever and more. 1 day agoTHE country should start securing bivalent vaccines to boost efforts to protect Filipinos from Covid-19 and keep the economy afloat Go Negosyo founder Jose Maria Joey.
The vaccine is authorised for use as a booster dose among those aged over 18 who have not received a third or fourth dose of Covid vaccine. The vaccines are called bivalent COVID-19 vaccines because they contain both components. Once this process is complete the new vaccines should be available shortly afterward.
The bivalent is designed to. Offer better protection against the more recent Omicron variants BA4 and BA5. Pfizers bivalent booster is authorized for those 12 or older while Modernas.
1 day agoThe Moderna bivalent vaccine generates a modestly higher level of antibody response against multiple SARS-CoV-2 Omicron subvariants approximately 16-19 times. Health Canada approved on Thursday the use of the Pfizer-BioNTech COVID-19 booster vaccine that targets the BA4 and BA5 strains of the Omicron variant.
Covid 19 Bivalent Vaccine Boosters Sept 24 2022

Fda Recommends Omicron Bivalent Covid 19 Boosters For Fall Infectious Disease Special Edition
Singapore Grants Interim Approval For Moderna S Bivalent Covid 19 Booster Vaccine Reuters

U S Health Agencies Recommend First Omicron Booster Shot Time
Covid 19 Bivalent Vaccine Campaign Launches Thunder Bay District Health Unit

Covid 19 Bivalent Booster Available Mercer County Ohio Health District

Eu Oks Bivalent Covid 19 Vaccines From Pfizer Biontech Moderna Pink Sheet
Covid 19 S Bivalent Boosters 10 Questions Answered Md Anderson Cancer Center
What You Need To Know About Bivalent Boosters For Covid 19 Hub
Vaccines Boosters Fishers In Official Website

Clinical Guidance For Covid 19 Vaccination Cdc
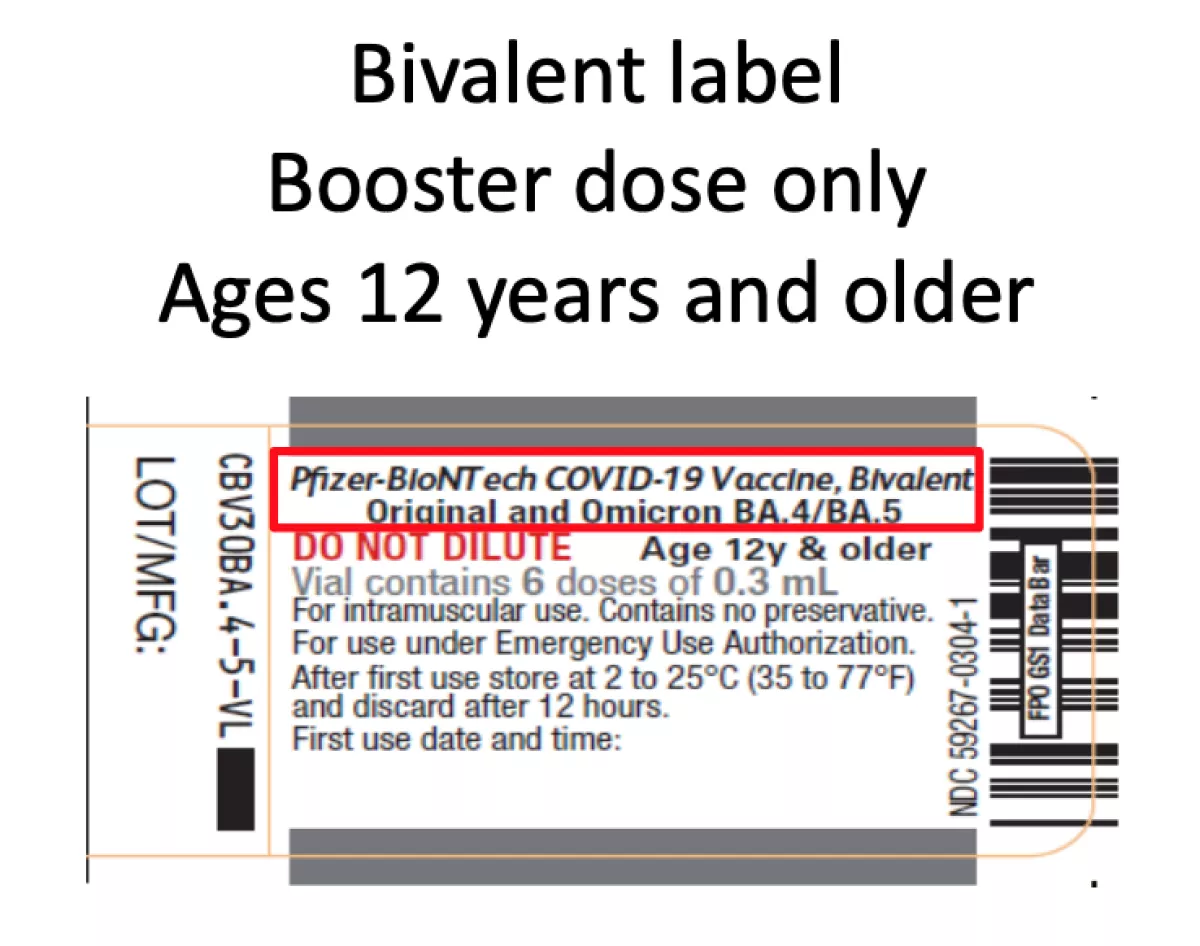
Alarm Over Risk Of Mixing Up Booster And Conventional Vaccine Los Angeles Times
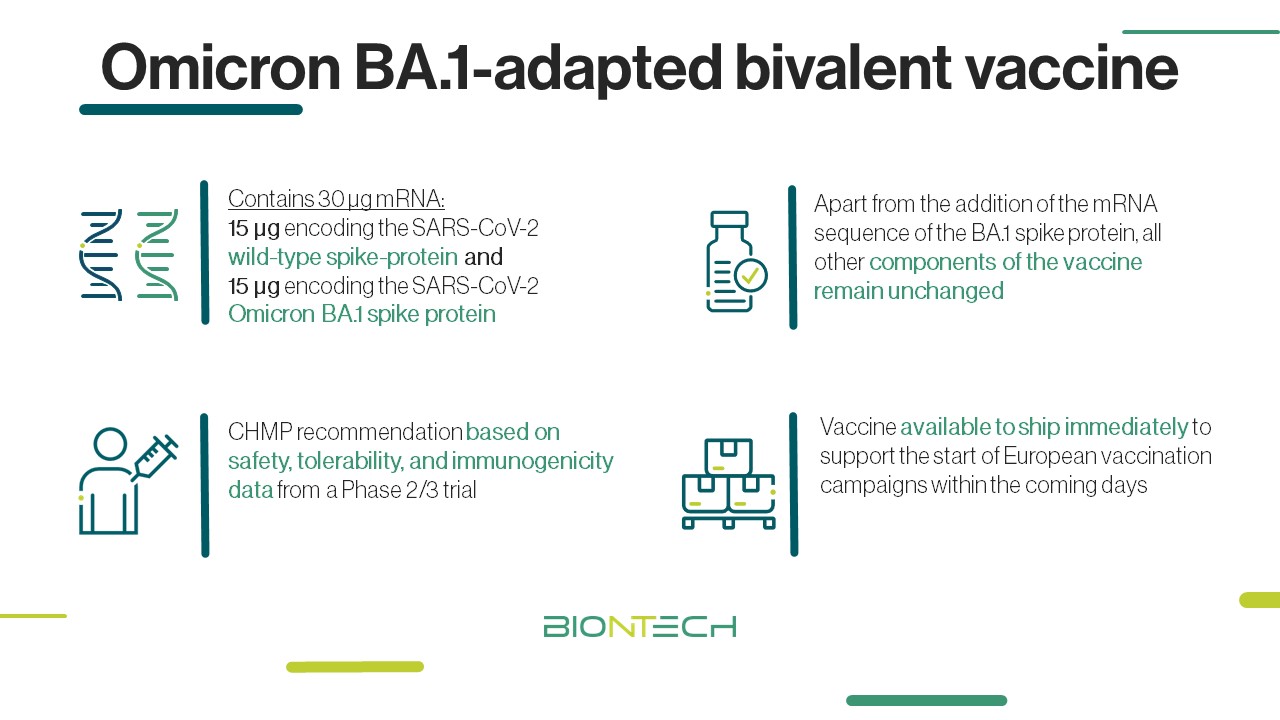
Biontech Se On Twitter Omicron Ba 1 Adapted Bivalent Vaccine Update Our Ba 1 Bivalent Covid 19 Vaccine Was Recommended For Conditional Marketing Authorization By Ema News Committee For Medicinal Products For Human Use As A Booster
/cloudfront-us-east-1.images.arcpublishing.com/gray/DFXC4VVIYJHHZLYDRKPUJI7CBA.jpg)
Pike County Health Department To Offer Bivalent Covid 19 Vaccine

Pfizer Seeks Ok Of Updated Bivalent Covid 19 Vaccine Booster For Fall Mint

Bivalent Covid Vaccines Are Here What Happens To The Existing Vaccines Cbc News

Covid 19 Bivalent Vaccine Booster Available Molokai Health Center

Covid 19 Bivalent Boosters Health Mil

Covid 19 Bivalent Vaccine And Flu Vaccine Now Available At Pat Walker Health Center University Of Arkansas